The Nitrogen Cycle
So far we’ve covered two essentials for life: water (for its flowing and carrying properties over a wide temperature range), and photosynthesis (for turning light energy into food which then flows up the food chain in an ecosystem). But no photosynthesis, whether oxygenic or anoxygenic, could occur without the nitrogen cycle, our topic for today.
Let’s begin!
The nitrogen cycle (on land) is this:
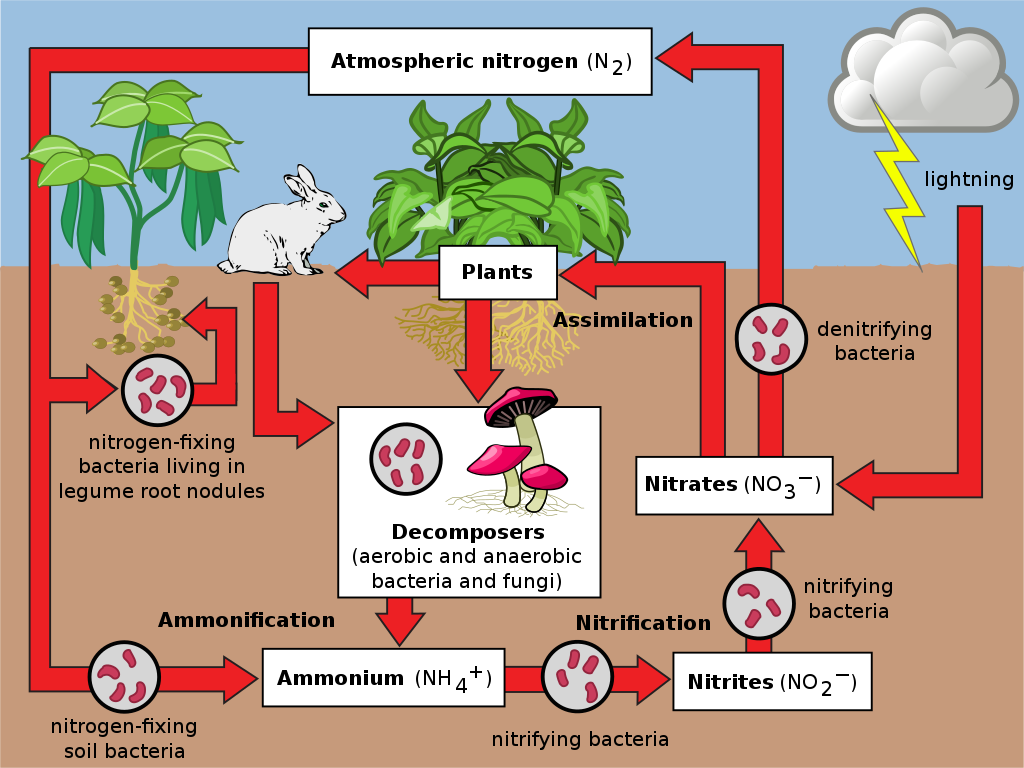
File:Nitrogen Cycle.svg: *Cicle_del_nitrogen_de.svg: *Cicle_del_nitrogen_ca.svg: Johann Dréo (User:Nojhan), traduction de Joanjoc d'après Image:Cycle azote fr.svg.derivative work: Burkhard (talk)Nitrogen_Cycle.jpg: Environmental Protection Agencyderivative work: Raeky (talk)derivative work: Hattiel [CC BY-SA 3.0 (https://creativecommons.org/licenses/by-sa/3.0)]
The marine nitrogen cycle is similar:
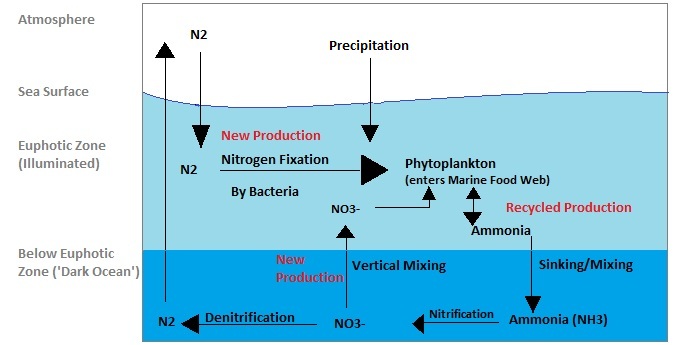
Attribution: Treachang [CC BY-SA (https://creativecommons.org/licenses/by-sa/3.0)]
Let’s start at the top with atmospheric nitrogen, an inert (non-reactive) gas made of two nitrogen atoms joined together with a very strong triple bond. We could show this visually as N≡N or (more commonly) write this as N2.
Nitrogen gas makes up 78% of the air we breathe, and yet it is inaccessible to the vast majority of organisms including us! It takes more energy than most organisms can harness to break that strong triple bond and release each nitrogen atom as a biologically reactive and accessible nitrogen ion (N3-).
Enter good old microbes — specifically, the nitrogen fixers. The scientific word for nitrogen fixer is diazotroph [’nourishment from two nitrogen (atoms)’]. You may know diazotrophs as the Rhizobium sp. bacteria associated with legume nodules, but there are free-living soil microbes too. Many anoxygenic photosynthesising bacteria are also nitrogen fixers and an important part of the nitrogen cycle in oceans.
Diazotrophs convert (or ‘fix’) atmospheric nitrogen into a nitrogen-containing compound called ammonia (NH3). This is done via enzymes (biological catalysts) called nitrogenases, the only group of molecules able to break nitrogen gas biochemically.
There is one non-biological agent with enough energy capable of also breaking that triple bond — lightning. Nitrogen can then react with oxygen in the atmosphere to produce nitrogen oxides (NO or NO2), which may further react with water to form nitrous acid (HNO2) or nitric acid (HNO3). These compounds can enter soil, where they dissociate and form the nitrate ion, NO3-, which is a form of nitrogen available to plants. Lightning fixes about half the nitrogen each year that diazomorphs fix.
Thus, biologically-fixed nitrogen begins as ammonia; non-biologically-fixed nitrogen begins as nitrogen oxides.
Ammonia (NH3) is a poisonous gas and needs to be converted into ammonium (NH4+) by another process called ammonification before continuing in the nitrogen cycle. This action is again microbial.
Ammonification is also an important step in the recycling of plant and animal matter by decomposers. A soil’s surface invariably contains dead animals and plants, and animal wastes such as urine. These contain organic nitrogen, that is, nitrogen combined with carbon. Some fungal and bacterial species are the decomposers that break that organic nitrogen down into ammonium through ammonification.
Enter now the nitrifying bacteria. Through a process called nitrification, ammonium is first converted into nitrite (NO2-), and then nitrate (NO3-), which is then uptaken by plants. We’ve now ended up with the same end product biologically as was produced non-biologically, though with more steps — still a very impressive feat considering those little microbes have had to (collectively) match the phenomenal energy in a lightning bolt!
The final step which completes the cycle is the return to the atmosphere of nitrogen gas. This process is done by denitrifying bacteria. These bacteria are facultative anaerobes, which means they can live in both aerobic (with oxygen) and anaerobic (without oxygen) environments, but the denitrifying process is only done anaerobically. As aerobes take in oxygen and release carbon dioxide, and oxygenic photosynthesisers take in carbon dioxide and release oxygen, anaerobic denitrifiers takes in nitrate and release nitrogen gas.
So why is the nitrogen cycle so important to life? Because it is the only way nitrogen becomes biochemically available to life, and while water is essential for life to even exist, all life as we know it, including photosynthesis would come to a slow end should the nitrogen cycle break.
Nitrogen is needed by all living things to make DNA, RNA, amino acids, proteins, and enzymes (which are also proteins), and because most organisms cannot break that strong triple bond to make nitrogen biochemically available for themselves, the nitrogen fixers must do that on their behalf. Otherwise, nitrogen is simply an inert gas occupying most of the atmosphere.
But once that triple bond is broken, nitrogen atoms readily flow through the ecosystem enabling growth (via protein and enzyme production), and reproduction (via RNA and DNA formation). Other microbes keep that flow in circulation by recycling nitrogen back into the environment via decomposition and denitrification.
Should the nitrogen cycle cease, nitrogen gas would start accumulating in the air and oceans. Both land and water photosynthesisers would deplete their respective environments of nitrates, and cease to grow and photosynthesise. When they die and are not replaced, those that feed on them will eventually run out of food and die, and so on up the food chain.
While the presence of water is the absolute foundation for all life on Earth, and photosynthesis starts the ball rolling by enabling all life to eat one way or another, it is the nitrogen cycle that keeps that ball rolling. While lightning can contribute to the cycle by creating nitrogen oxides, it can neither maintain the cycle nor complete it. It is microbes alone that are crucial in every step of the nitrogen cycle from beginning to end.
Next week I’d like to explore a bit more the role of microbes in other aspects of life, including a very important one involving leaves that may well shock you!
About the Author
BSc(Hons), U.Syd. - double major in biochemistry and microbiology, with honours in microbiology
PhD, U.Syd - soil microbiology
Stumbled into IT and publishing of all things.
Discovered jujube trees and realised that perhaps I should have been an agronomist...
So I combined all the above passions and interests into this website and its blog and manuals, on which I write about botany, soil chemistry, soil microbiology and biochemistry - and yes, jujubes too!
Please help me buy a plant if you found this article interesting or useful!