My Take on the Carbon Dioxide Narrative: Part 2 Extended
Introduction
Part 3 on the Sun and climate is coming — slowly! — but in the meantime I just had to include a snippet from this most timely two-year-old talk by Dr Willie Soon which came my way just recently (thanks Phil!).
Dr Soon’s entire talk is highly recommended and can be viewed in the following video:
but this post will cover about three and a half minutes of it. Those few minutes perfectly add to the discussion in the last post on the incorporation of carbon dioxide (CO2) as calcium carbonate by marine life.
Background
The previous post opens with a lament that the vast bulk of ‘climate change’ science focuses on the abiotic sciences (for example atmospheric chemistry, atmospheric physics, meteorology, climatology and oceanography), whilst completely ignoring anything within the biosphere.
This is disingenuous at best and outright subterfuge at worst, and it was a refreshing breath of fresh air for Dr Soon, a geophysicist, to mention the effects of CO2 on marine life at all.
What he said — and showed — briefly assumes a bit of chemistry to fully understand, so I’ll reproduce what I wrote previously before covering his bit.
The first multicellular life was soft and jellyfish-like, and lived only in the watery environments of the early Cambrian Period. Partway into the Cambrian, around 500 million years ago, the first shell evolved, and this natural armour-plating proved an immediate and huge evolutionary advantage.
These shells were made from calcium carbonate (CaCO3), which formed from dissolved calcium ions (Ca2+) and carbonate ions (CO32-) in the oceans.
The reaction is:
Ca2+ + CO32- → CaCO3The carbonate ions themselves originated from dissolved CO2 in the oceans.
Dissolved CO2 reacts with water (H2O) to produce the weak acid carbonic acid (H2CO3). This molecule deprotonates (loses a proton, the H+ ion) to form hydrogencarbonate (also called bicarbonate, HCO3-) when in water. Hydrogencarbonate in turn deprotonates to the carbonate ion (CO32-), and all three molecules protonate and deprotonate to establish an equilibrium in water:
H2CO3 + 2H2O ⇌ HCO3- + H3O+ + H2O ⇌ CO32- + 2H3O+
Chemical equilibrium is established when a reaction occurs both forward and in reverse, and when products and reactants do not change their respective concentrations over time. Both the forward and reverse reactions occur at the same rate and there does not appear to be any change in the properties of the system.
A change to the concentration of a reactant or product will cause the equilibrium to shift either left or right until a new balance and equilibrium results.
If conditions change such that all reactants are fully consumed, then the equilibrium is said to shift fully to the right.
If conditions change such that reactants don’t react (no products form), then the shift is said to be fully to the left.Let’s add CO2 and water to the beginning of the above equilibrium:
CO2 + 3H2O ⇌ H2CO3 + 2H2O ⇌ HCO3- + H3O+ + H2O ⇌ CO32- + 2H3O+And let’s refer back to this other from above:
Ca2+ + CO32- → CaCO3Does it make sense that if animals which are making calcium carbonate shells are becoming increasingly successful, that evolution will drive more and more species to have such shells?
Does it also make sense for these species to require more and more Ca2+ and CO32- ions to be available as their populations increase?
Can you see that if the carbonate ion (CO32-) products on the right of the first equation go on to react with calcium ions to form calcium carbonate in the second reaction, that they have been removed from that first equation?
Can you see that a removal of this product from the first equation shifts this equilibrium to the right, as there is now less carbonate to react in the reverse reaction?
Can you see that for more carbonate ions (CO32-) to form on the right, for species to avail themselves of, that more CO2 must enter the water so as to drive the equation to the right?
Can you see how the evolution of calcium carbonate shells could be responsible for the permanent removal of tonnes and tonnes of CO2 from the atmosphere?
Now back to Dr Soon.
Of Lobsters, Crabs and…Hydrochloric Acid??
If you ever fell for the lie that rising CO2 levels will acidify the ocean, I just showed how that just is not so — a significant chunk of the marine biosphere will ensure that it is converted to (the alkaline) CaCO3.
That discussion above concerned the marine life which incorporate CaCO3 into shells, as that was what the talk by Dr Patrick Moore I drew on in the last post focused on. Dr Soon mentions another group of marine life making use of CaCO3 — those with exoskeletons, made of chitin which incorporates, yes you guessed it, CaCO3!
As with the previous posts on this topic, I shall include screenshots from Dr Soon’s talk with direct links to the timestamps they were taken at.
He begins with (his words) “a new scare" — ’Osteoporosis of the Sea’!

Source: https://youtu.be/1zrejG-WI3U?t=1218
This was new to me! Apparently all this acid forming in the oceans is dissolving creatures away!
And who or what is affected?

Source: https://youtu.be/1zrejG-WI3U?t=1226
Why, the very creatures which, as shown with chemistry above, make sure the oceans aren’t acidifying in the first place!
And should you take the word of a (in Dr Soon’s own words: “so-called") marine biologist over a geophysicist, please peruse the following two slides.
Here is a lobster exposed to the same CO2 level in seawater as in Earth’s atmosphere — 400 ppm (parts per million), or 0.04%:
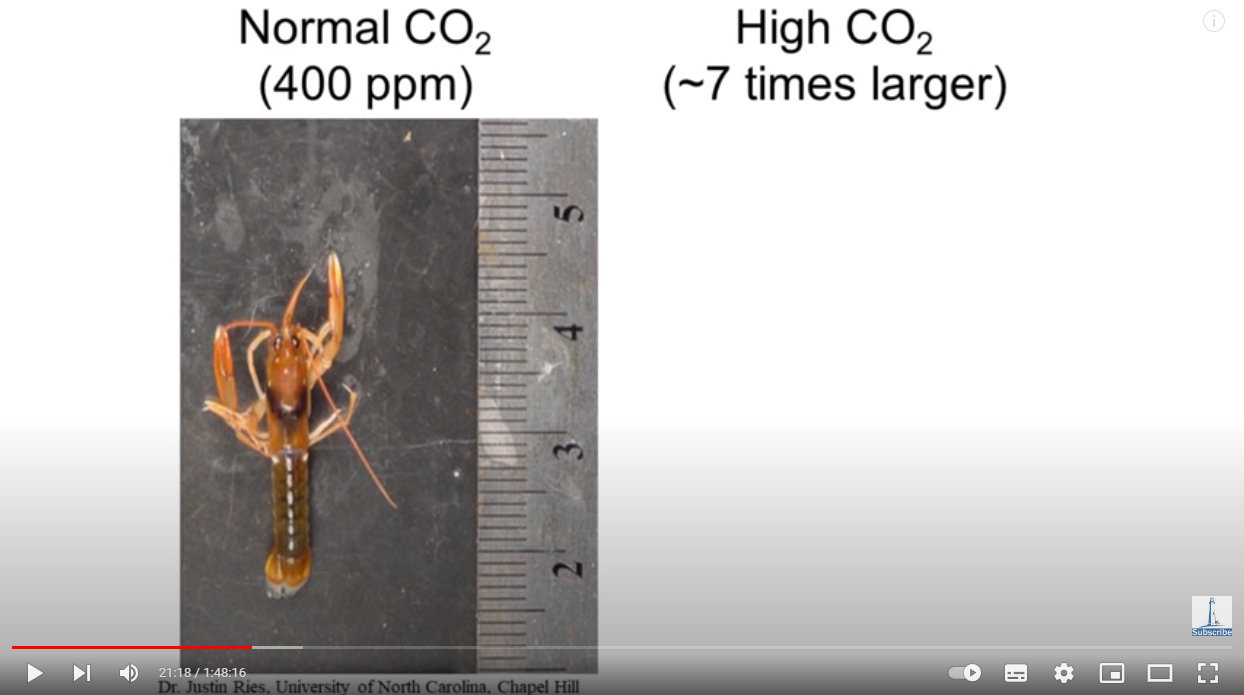
Source: https://youtu.be/1zrejG-WI3U?t=1276
And at about 2,800 ppm, or 0.28%:
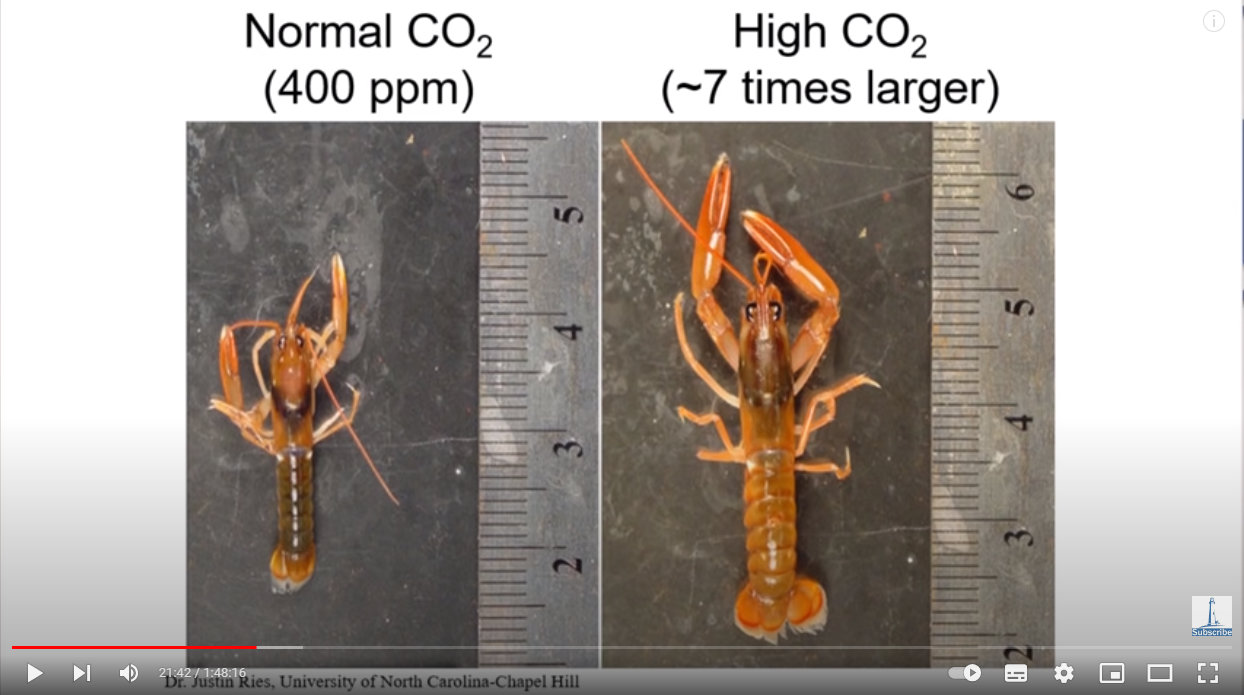
Source: https://youtu.be/1zrejG-WI3U?t=1302
The lobsters melted grew bigger!
As did crabs:
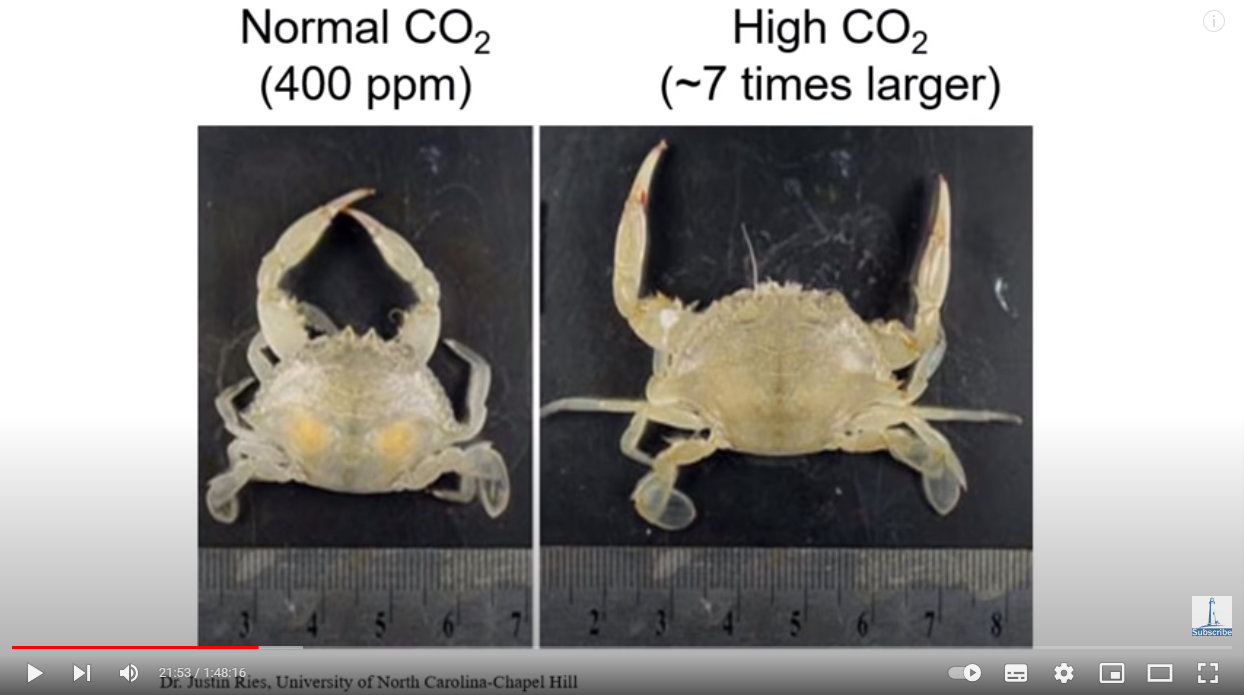
Source: https://youtu.be/1zrejG-WI3U?t=1313
All that extra CO2 in their environment didn’t kill them, but rather enabled them to incorporate more into their exoskeletons as calcium carbonate, enabling larger lobsters and crabs with larger exoskeletons. CO2 is a nutrient. CO2 doesn’t harm life, it enriches it.
But what about all those pictures of dissolving shells that are used as proof of rising ocean acidity, like these:
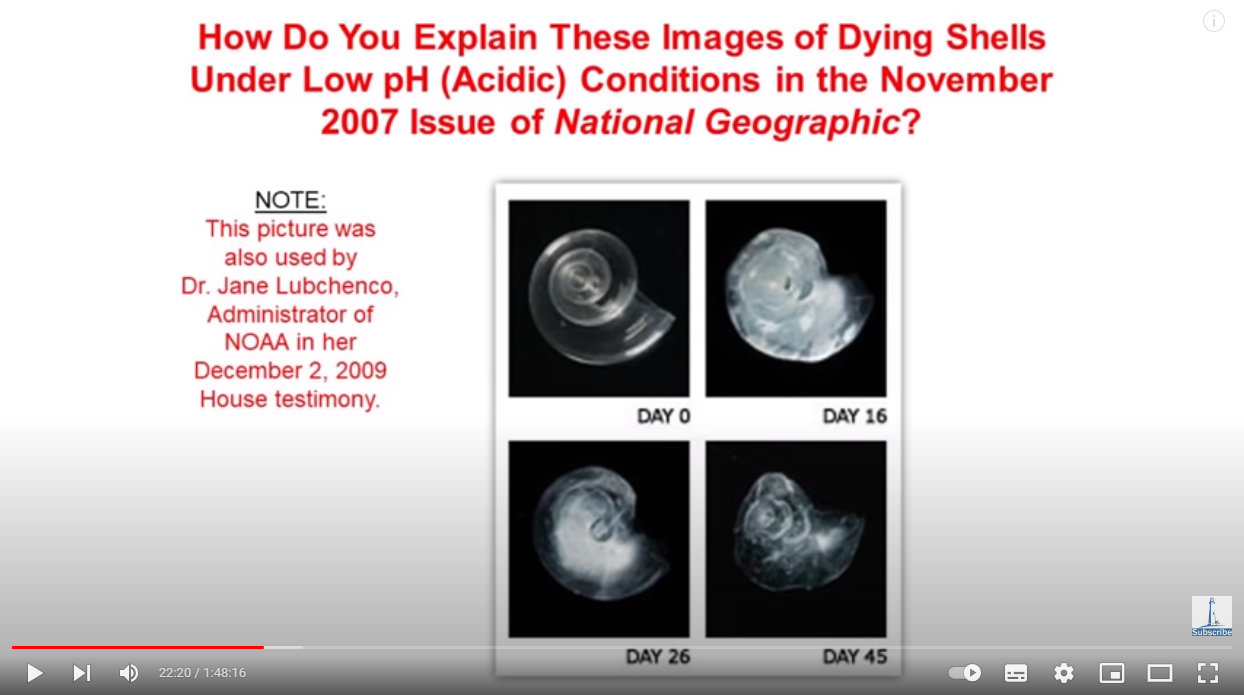
Source: https://youtu.be/1zrejG-WI3U?t=1340
Well, unlike the lobster and crab experiments, which took months to do and required the bubbling through water of different CO2 concentrations and measuring subsequent changes in pH and carbonate levels, these shell-dissolving experiments…used hydrochloric acid to artificially raise acidity and raise it quickly, and had nothing whatsoever to do with simulating a natural environment!

Source: https://youtu.be/1zrejG-WI3U?t=1402
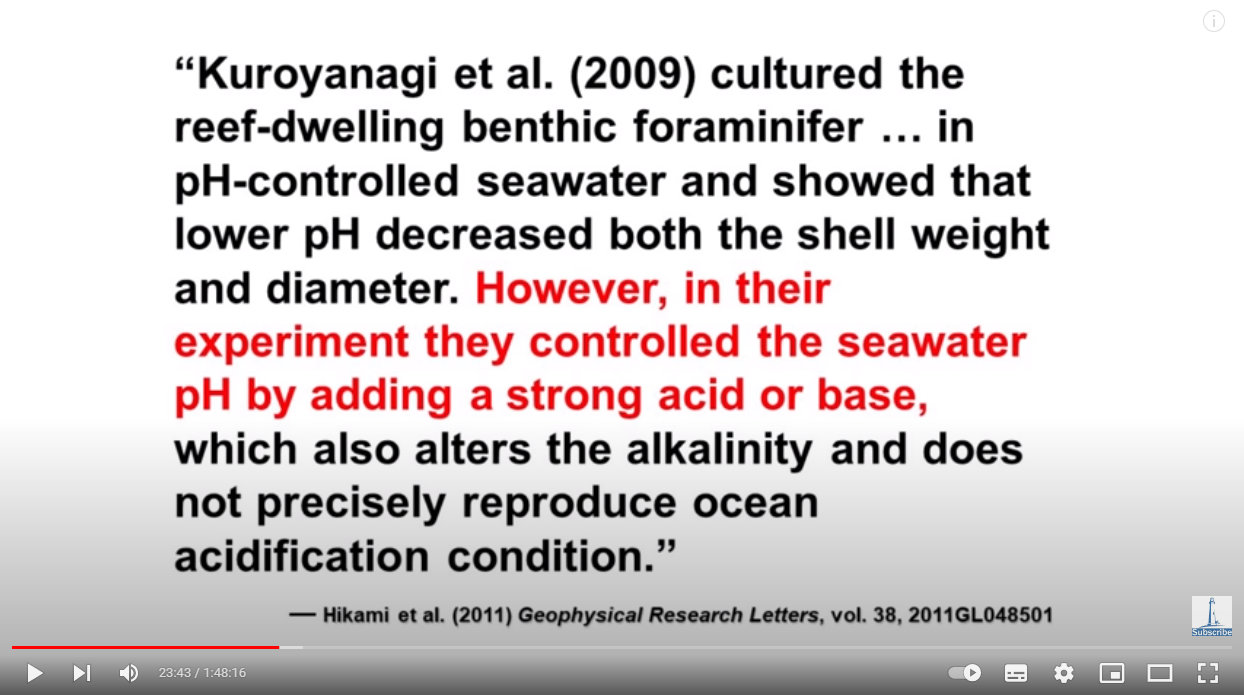
Source: https://youtu.be/1zrejG-WI3U?t=1423
Conclusion
What is there to say that isn’t already so obvious?
It is really easy to show through honest experimentation that CO2 is not acidifying the oceans and is not destroying life. See also this shown-before image on how CO2 enriches life outside of the oceans:

Attribution: unknown, but slide is viewable on the video 'NASA Engineer Tom Moser Reveals the Truth About Climate Science" at this timestamp: https://youtu.be/1-gHWcrCK7w?t=788
It takes disingenuity and deceit to push fearmongering and a false narrative. Why do this?
As I said at the beginning of this carbon dioxide narrative:
Any time you hear yet more fear pοrn concerning ‘global warming’ or ‘carbon emissions’ or ‘rising temperatures’, just ask yourself cui bono? (who benefits?). Odds are the ’solution’ being proposed won’t benefit you in any way, but will benefit governments via taxpayer money, regulations, new laws, and gross inconveniences and changes to ordinary people’s daily lives.
Banning gas stoves, anyone?

Attribution: unknown
About the Author
BSc(Hons), U.Syd. - double major in biochemistry and microbiology, with honours in microbiology
PhD, U.Syd - soil microbiology
Stumbled into IT and publishing of all things.
Discovered jujube trees and realised that perhaps I should have been an agronomist...
So I combined all the above passions and interests into this website and its blog and manuals, on which I write about botany, soil chemistry, soil microbiology and biochemistry - and yes, jujubes too!
Please help me buy a plant if you found this article interesting or useful!