Soil Properties
Soil properties of most interest to gardeners and horticulturalists are colour, texture, mineral content, structure, and chemistry. Each of these could be lengthy posts in their own right (and maybe they will be, later!), but the following overviews should get you up to speed on the concepts.
Colour
Soil colour mostly comes down to two things: the type and amount of iron oxides present, and the amount of organic matter present.
Iron oxides are compounds of iron (Fe) and oxygen (O) — common rust (Fe2O3) is the best-known example. Iron oxides form in soils in the presence of air and water and their colours range from yellows, through to oranges, browns and reds. Most soils contain mixtures of different types, the proportions of which determine the overall colour.
Soil colour can provide clues as to drainage and aeration, particularly of the subsoil. A uniform brown or reddish-brown colour extending into the subsoil signifies a well drained and aerated soil — the colour indicates iron oxides, which indicates the presence of air and water oxidising iron at those depths.
Yellow shades indicate moderate waterlogging, and extremely waterlogged soils consist of the ‘gley’ (not a typo!) colours. ‘Gley’ colours (dark greys, green-greys, green-blues and blacks) indicate the presence of iron hydroxy-carbonates (’green rusts’) which form in very wet and air-poor environments.
Organic matter darkens soils, and topsoils, where organic matter accumulates, are usually darker than subsoils. Organic matter or certain minerals like ilmenite can be behind the colour of black soils. Soils made of lighter greys and whites are a sign of no organic matter and significant leaching over extended periods.
Texture
Soil is made up of differently-sized particles, summarised here:
| Particle | Diameter (mm) |
| Clay | less than 0.002 |
| Silt | 0.002 - 0.02 |
| Fine sand | 0.02 - 0.2 |
| Coarse sand | 0.2 - 2 |
| Gravel | greater than 2 |
The proportions of each of these five in soil determines a soil’s texture, or ‘feel’. The word ‘feel’ comes from the technique of working a moistened sample between fingers and thumb. Soils with a lot of clay will become sticky and easily from ribbons when rolled, while soils with a lot of sand will feel gritty and barely form a workable ball. Many other soils will fit somewhere within this range.
A soil laboratory would of course analyse a soil sample more accurately, but as a field technique this is a great way to get, pardon me!, a feel for your soil. We’ll actually go over how to do this technique, as well as some other simple soil experiments you can easily do at home in an upcoming post.
The following Soil Texture Tirangle diagram shows how soils are classified based on their proportions of clay, sand and silt.
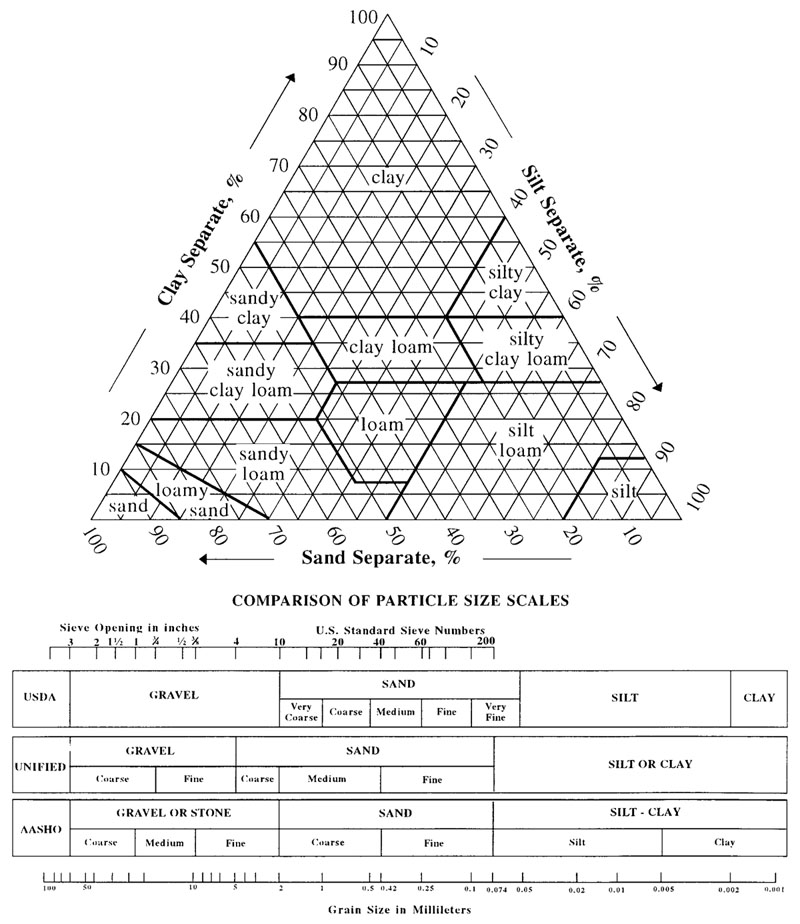
Attribution: USDA Soil Survey Division Staff [Public domain]
A soil’s composition and texture reveals much about its properties and characteristics, though this is not definitive, as the mineral content and organic matter present (or not) also has an influence. General statements can still be made however.
(Go here for details on how to assess your own soil texture and composition!)
For example, soils high in sand but low in clay and silt tend to drain easily. This can be to your advantage if watering with, say, bore water of moderate salinity, as the salts are likely to leach away rather than build up to toxic levels. This can also work against you as sands are not able to hold organic matter.
Too much clay, with little sand and silt, can result in heavy, sticky, poor-draining and waterlogged soils when wet, and hard, cracked surfaces when dry. Clay soils tend to be rich in organic matter though, as they are particularly good at holding nutrients.
Silt’s properties are partway between those of sand and clay. Its drainage ability is less than sand’s but higher than clay’s. On the other hand, it can hold onto more nutrients than sand, but not as much as clay can.
An ideal soil, from a plant-growing point of view, is one of a roughly balanced mix of sand, silt and clay — the loams.
Mineral Content
Sand minerals in soil have small surface areas relative to their volume, low water retention ability, and low chemical activity. These properties account for their inability to hold nutrients in the soil. Silt minerals also have small surface areas relative to their volume and low chemical ability. Soils high in silt can compact under heavy traffic, which impacts water and air movement.
Clay minerals are the ones that most influence soil properties. These minerals exist as very small rod and plate-like crystals with very large surface areas relative to their volume. This and the fact they are also very chemically active is why they hold onto water and nutrients so well.
Structure
Soil structure refers to how the solid parts (peds) and the spaces (pores) are arranged.
A good soil structure is one with fine soil particles (crumbs) distributed somewhat evenly amongst spaces through which air, water and plant roots can travel smoothly. A high organic matter content, roots growing through the soil, and earthworm activity all contribute to the creation of channels and pores through which air and water can percolate. If soil is left undisturbed these form a positive feedback loop and both structure and organic matter increase still further over time. Better flow of air and water increases root growth and attracts earthworms and other biological activity from arthropods, insects and microorganisms. These have a combined effect of breaking larger soil peds into smaller ones, creating still more organic matter, and forming still more pores and channels which further increases air and water movement, attracts further root growth and biological activity, and so on.
A poor structure is characterised by soil peds compacted into clods with poor air and water percolation. High traffic can exacerbate this compaction and impede air, water and root movements. Excessive digging of soil will destroy any channels formed by earthworms and possibly lead to clod formations, especially in more clayey soils. Both of these can be overcome, and the structure improved, by incorporating high amounts of good quality compost and/or manure into the soil and leaving it to resettle and reform.
Chemistry
Soil chemistry has a major effect on plant growth. It is the means by which minerals and organic matter are broken down in the soil and made accessible to plants. This chemical activity can be non-biological, biological, or a combination of both.
Much of this activity occurs because of the negatively-charged surfaces of clay minerals and humus particles. (Humus is the dark, spongy and jelly-like end result of organic matter decompostion.) The negatively-charged surfaces attract and hold positively-charged ions of calcium, potassium and magnesium, which may then be uptaken directly by plant roots or dissolve into the soil water for uptake that way. These negatively-charged surfaces also attract many microorganisms that break down organic matter.
We saw earlier how pH (essentially the chemistry of hydrogen ions in solution) affects the availability of nutrients:
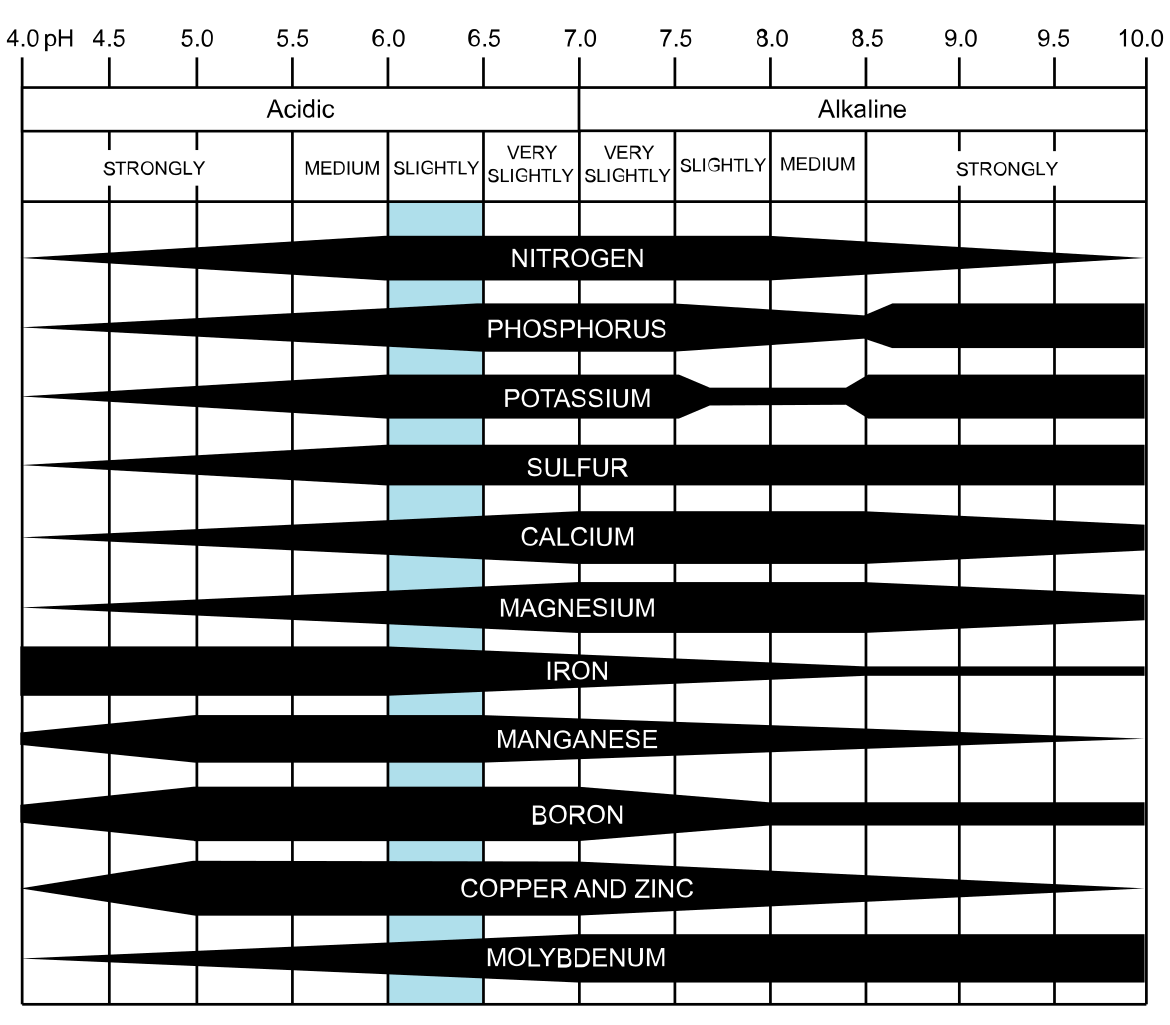
Attribution: CoolKoon [CC BY 4.0 (https://creativecommons.org/licenses/by/4.0)]
Lowering pH by applying large amounts of acidifying fertilisers such as ammonium sulfate or ammonium nitrate can lead to a molybdenum deficiency or a manganese toxicity. Raising pH by applying large amounts of lime may lead to iron and/or manganese deficiencies.
Nitrogen wouldn’t even be available to plants were it not for the action of soil microbes (and to a much lesser extent, lightning strikes) ‘fixing’ atmospheric nitrogen into nitrogenous compounds plants can uptake.
Chemical reactions can also act in deleterious ways. Excessive amounts of potassium can inhibit magnesium uptake, leading to a magnesium deficiency. The reverse also occurs, in that excessive amounts of magnesium can lead to potassium deficiencies. Excessive use of phosphatic fertilisers can cause zinc deficiencies even in zinc-rich soils. And soils with high levels of manganese can prevent Rhizobium bacteria from accessing the cobalt they need to fix nitrogen for their leguminous hosts.
About the Author
BSc(Hons), U.Syd. - double major in biochemistry and microbiology, with honours in microbiology
PhD, U.Syd - soil microbiology
Stumbled into IT and publishing of all things.
Discovered jujube trees and realised that perhaps I should have been an agronomist...
So I combined all the above passions and interests into this website and its blog and manuals, on which I write about botany, soil chemistry, soil microbiology and biochemistry - and yes, jujubes too!
Please help me buy a plant if you found this article interesting or useful!