Essential Macronutrients: Magnesium (Mg)
Magnesium (Mg) in soil ranges from about 0.05% in sandy soils up to 0.5% in clay soils. It is more prevalent in clay soils as these contain very weatherable magnesium-containing minerals. Some clay minerals themselves contain magnesium. Magnesium may also be present in some soils as magnesium carbonate (MgCO3) or dolomite [calcium magnesium carbonate, CaMg(CO3)2]. Arid and semi-arid soils may contain large amounts of magnesium as magnesium sulfate (MgSO4).
Availability to Plants
Owing to the presence of magnesium in many soil minerals, its availability to plants is similar to that of potassium, which is also a component of minerals. There is a non exchangeable form (locked away in clay and other particles), an exchangeable form (on the surfaces of colloids), and a form dissolved in the soil water. These three states are in equilibrium, and the largest component is the non-exchangeable magnesium. Of this non-exchangeable magnesium, some is released — as with potassium — when clay particles expand and separate when wetted.
Exchangeable magnesium is about 5% of the total soil magnesium, and this amount along with that in the soil water is the largest contributor of available magnesium to plants. Of the total cation exchange capacity, magnesium is about 4 – 20%. This is much lower than that of calcium (80%), but higher than potassium (up to 4%).
Magnesium ions (Mg2+), as with calcium ions (Ca2+), are readily leached from soil. In many soils, the rate of weathering of magnesium-containing minerals can balance this removal, but in sandy soils with little clay, removal by leaching will predominate and magnesium will be found in higher concentrations in the subsoil.
The Australian soils which tend to be low in magnesium are those in high rainfall areas, and especially in the coarse-textured acid soils near the coast.
Magnesium in Biochemistry
A magnesium atom is at the heart of every chlorophyll molecule and thus essential for both the synthesis of chlorophyll as well as in photosynthesis. Below is the structure of chlorophyll a, and the structures of the other (similar) chlorophyll variants are here.
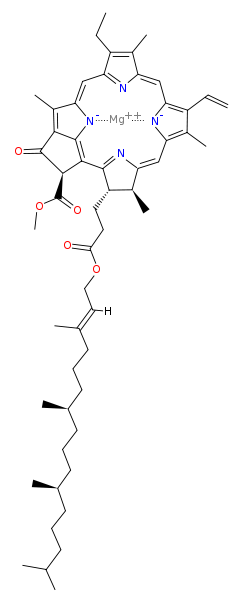
Attribution: David Richfield [Public domain]
Magnesium also features in many enzyme reactions, and in all the ones that use or synthesise adenosine triphosphate (ATP), as ATP must bind to magnesium to become biologically active. Mg2+ also binds to and strengthens the structures of DNA and RNA. Magnesium, as with calcium, is also important in strengthening cell walls and in cell membrane stability and permeability.
Magnesium Deficiency Symptoms
Mg2+ ions have a higher concentration in soil water than potassium ions (K+), but uptake of Mg2+ is lower than of K+. An excess of K+ can thus severely interfere with the uptake of magnesium, as can other cations (positively-charged ions) such as ammonium (NH4+).
Magnesium uptake is also reduced in acidic soils, but this is not so much due to the increase of H+ ions, but because the increased availability of aluminium ions (Al3+) interferes with Mg2+ uptake.
Magnesium deficiency symptoms can vary between species, but there are also general similarities. For example, as with potassium ions (K+), Mg2+ is very mobile within a plant, and can move from older to younger leaves. Deficiencies will show up in older leaves first as a result.
Interveinal (between the veins) yellowing occurs as chlorophyll breaks down and is not replaced (chlorosis). Necrotic (dead) spots may appear in these yellow regions in extreme deficiencies. Plants exposed to strong sunlight may take on a wilted appearance, and individual leaves may become stiff, brittle, and fall off prematurely.
Magnesium Toxicity Symptoms
Magnesium toxicity is rare, and it’s more likely that high amounts of magnesium will induce a calcium deficiency rather than a magnesium toxicity, by interfering with the uptake of Ca2+ ions.
There is actually a balance between Mg2+, Ca2+ and K+ ions and their uptake from soil, due in part to their positions on the Periodic Table. These are all positively-charged elemental ions close to each other on the Table, and have similar properties because of this.
Magnesium and calcium are elements 12 and 20 respectively on the Table. Both are in the Group 2 column, meaning they both form 2+ ions. Magnesium is immediately above calcium, and is a much smaller atom, with 12 protons compared to calcium’s 20. In very simple terms, this makes a Mg2+ ion much easier to uptake than a Ca2+ ion.
Potassium is element 19, with just one proton less than calcium’s 20. This makes it only slightly smaller in size, but it is a Group 1 element with a charge of 1+ compared to calcium’s 2+. Potassium ions are rapidly taken up by plants while that of calcium ions is slow. Potassium ions, as mentioned above, can also interfere with the uptake of magneisum.
It might be worth discussing this balance between the three, and the chemistry, in deeper detail in a separate post. But certainly the take-home message here is that there is a balance, and that growth of plants will be affected should any of the three nutrients be significantly out of balance.
About the Author
BSc(Hons), U.Syd. - double major in biochemistry and microbiology, with honours in microbiology
PhD, U.Syd - soil microbiology
Stumbled into IT and publishing of all things.
Discovered jujube trees and realised that perhaps I should have been an agronomist...
So I combined all the above passions and interests into this website and its blog and manuals, on which I write about botany, soil chemistry, soil microbiology and biochemistry - and yes, jujubes too!
Please help me buy a plant if you found this article interesting or useful!