 From Soil to Fruit
From Soil to Fruit- To Fruit
- Photosynthesis in Plants
- Visible Light and Photosynthetic Pigments
Visible Light and Photosynthetic Pigments
Photons and the Electromagnetic Spectrum
‘Photons’ are commonly thought of as the particles of light, but they are actually the particles of radiation, of which light is just one form.
Radio waves, microwaves, infrared waves, visible light waves, ultraviolet waves, x-rays and gamma rays are all forms of radiation, each of which can be defined by either their wave frequency or their wavelength.
The wave frequency is the number of times a wave cycles per second. ‘One cycle per second’ is a unit of measurement called the hertz (Hz).
The wavelength is the distance between two peaks or troughs of a wave, and the shorter the wavelength, the higher the energy.
These frequencies and wavelengths form a continuum called the electromagnetic spectrum (Fig. 1):
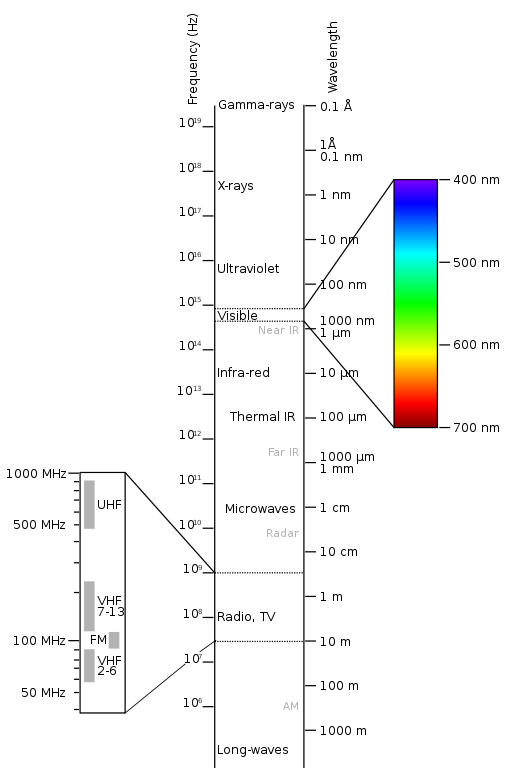
Attribution: !Original: PenubagVector: Victor Blacus, CC BY-SA 3.0 , via Wikimedia Commons
At one end of this spectrum are the very long radio wave photons, with a frequency of 3 Hz and a wavelength of 100,000 km — longer than the Earth’s diameter — and beyond. At the other end are the very short gamma ray photons, with a frequency of 300 exahertz (EHz, 1018 Hz) and a wavelength of 1 picometre (pm, 10-12 m).
Fig. 2 is a good summary of the electromagnetic spectrum’s properties:
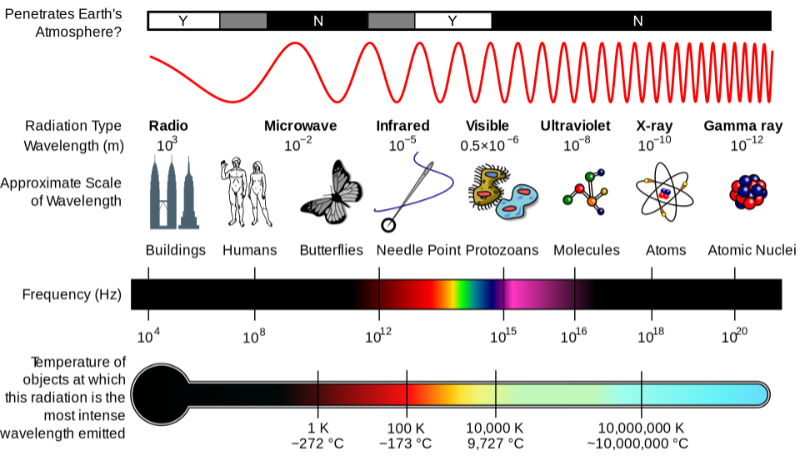
Attribution: Inductiveload, NASA, CC BY-SA 3.0 , via Wikimedia Commons
Visible Light
What we call ‘visible light’, also known as the ‘visible spectrum’, occupies a very thin band of the electromagnetic spectrum, and is defined as the range of wavelengths a typical human eye can detect (though some people may be able to see slightly either side of this defined range). We see this entire band as white light collectively, and ‘the colours of the rainbow’ when separated into still smaller bands.
It’s worth noting that this ‘visible light’ is nothing more than an arbitrary range that our eyes can detect — for example, many, if not all birds readily see in the ultraviolet range, which is part of their ‘visible light spectrum’ but not ours.
Photons within our visible spectrum range from frequencies of 400 – 790 terahertz (THz, 1012 Hz) and wavelengths of 380 – 700 nanometres (nm, 10-9 m).
Our eyes detect the lower frequency/longer-wavelength end of the visible spectrum as red light, and the higher frequency/shorter-wavelength end as violet light. Infrared (‘below-red’) radiation sits below visible red and ultraviolet (‘beyond-violet’) radiation sits above visible violet.
Just as our eyes are optimised for a specific range of frequencies within the electromagnetic spectrum, so too are the photosynthetic pigments.
Photosynthetic Pigments
A pigment is a substance which absorbs visible light. What isn’t absorbed is reflected, and it is the reflected wavelengths we see as colour. A substance which does not absorb any wavelength within the visible light spectrum reflects each one back, and we see it as white. Conversely, a substance which does absorb all wavelengths within this spectrum reflects none back, and we see it as black.
There are several photosynthetic pigments, spanning a range of colours, the colour of each dependent on the particular molecule and wavelengths it reflects.
Anoxygenic Photosynthetic Pigments
Anoxygenic photosynthetic pigments are called bacteriochlorophylls, of which there are eight known: bacteriochlorophylls a, b, c, cs, d, e, f, and g. These molecules are found only in phototrophic bacteria and archaea, most of which are anoxygenic photosynthesisers.* As anoxygenic photosynthesisers were the first photosynthesisers to appear on Earth, it’s possible that these bacteriochlorophylls are representative of the first photosynthetic pigments to ever appear on Earth.
* Some bacteriochlorophyll-containing microbes are phototrophs, but not autotrophs, and thus not photosynthesisers. There are no photosynthesising archaea.
No phototrophic microbe has all eight bacteriochlorophyll versions, but two or three at most.
Bacteriochlorophylls absorb wavelengths not absorbed by the oxygenic photosynthesisers, which may be a carry-over from the environment of early Earth. Bacteriochlorophyll g absorbs wavelengths at 670 and 788 nm, on either side of the red-infrared boundary of 700 nm. The other bacteriochlorophylls absorb even longer wavelengths, all in the infrared range of 788 nm – 1 mm, and the bacteriochlorophyll b of the purple bacteria can absorb even longer wavelengths into the microwave spectrum — but only just! Microwave wavelengths range from 1 mm – 1 m, and bacteriochlorophyll b absorbs those from 1020 – 1040 nm (1.02 – 1.04 mm).
Oxygenic Photosynthetic Pigments
These are the pigments found in plants, algae and cyanobacteria, though carotenes, xanthophylls and phaeophytins can also be found in some bacteria.
Carotenes
Carotenes are a group of related pigments responsible for the orange colour of carrots. We see them as orange hues as they absorb ultraviolet, violet, and blue light while reflecting orange, red and yellow light.
Xanthophylls
Xanthophylls are a group of yellow pigments which absorb and reflect similar wavelengths as the carotenes — xanthophylls and carotenes are very similar in structure and together make a larger group called the carotenoids.
Phaeophytins
Phaeophytin a is a grey-brown pigment and phaeophytin b is yellow-brown.
Chlorophylls
Chlorophylls are the best-known of all the photosynthesising pigments, and there are several versions as with the bacteriochlorophylls: chlorophyll a, b, c1, c2, d, and f.
Chlorophyll a is the most common and found in all oxygenic photosynthesisers, b is mostly in plants, c1 and c2 are in algae, and d and f are in the cyanobacteria. As cyanobacteria were the first oxygenic photosynthesisers, it’s possible that chlorophyll d and/or f are representative of the first chlorophyll molecules to have ever appeared on Earth.
Each chlorophyll molecule absorbs a range of wavelengths specific to it: chlorophyll a, for example, absorbs blue light from 400 – 450 nm and red light from 650 – 700 nm, while chlorophyll b absorbs blue light from 450 – 500 nm and red light from 600 – 650 nm.
The chlorophylls d and f specific to cyanobacteria hint at the evolution of cyanobacteria from the anoxygenic photosynthesisers, as these are the only oxygenic pigments capable of absorbing in the infrared range. Both absorb just outside visible red light, at around 710 nm.
The carotene, xanthophyll and phaeophytin pigments reveal themselves in autumn leaves when deciduous trees break down the chlorophyll so as to reabsorb its components from their leaves before shedding them. These non-green pigments were always there, but outnumbered and obscured by the amount of chlorophyll present.
Why Aren’t Leaves Black?
As we’ve seen, each oxygenic photosynthetic pigment type has its own wavelength range at which absorption is most efficient. The presence of several different pigments increases the total amount of visible light which can be absorbed.
Green and yellow light are the most abundant wavelengths in sunlight — the most amount of the sun’s energy is in the green region of 483 – 520 nm — and yet none of the photosynthetic pigments absorb in the green-yellow range. It is, after all, these wavelengths reflecting back which are responsible for the mass of green we see in nature.
Why are the most abundant wavelengths, in a sense, ‘wasted’ by oxygenic photosynthesisers? Why isn’t every wavelength made use of, which would make cyanobacteria, algae and plants black?
It turns out that there is no definitive answer to this, but quite a few theories!
One is that there is more sunshine than photosynthesisers know what to do with. The chemical reactions driven by photosynthesis only require so much energy, and absorbing the most energetic part of the visible spectrum would only lead to overheating. Not absorbing this high energy possibly protects against a plant version of sunburn.
On a similar note, absorbing too much energy may lead to the production of too many loose electrons, causing a phenomenon known as photoinhibition, and the production of tissue-damaging free radicals.
Another possibility is that, for plants especially, being able to respond to different levels of blue and red light as the seasons change could aid in growth, preparation for dormancy, or timing flowering correctly.
Another theory concerns evolution never resulting in optimal solutions, but rather in ‘good enough’ solutions. These pigments ‘just work’ and have done so for millions of years with no need for drastic changes. An offshoot of this concerns the probable water environment plants first developed in, where blue light is the most available. When plants moved onto land they then possibly developed the means to absorb red light which is more abundant in that environment.
My favourite theory, for what it’s worth, involves the cyanobacteria and chloroplasts. Cyanobacteria were the first oxygenic photosynthesisers to appear on Earth, and cyanobacteria, unlike the anoxygenic bacteria, contain chlorophyll. Chloroplasts are the chlorophyll-containing organelles in plant cells in which photosynthesis occurs.
There is much evidence to suggest that the first chloroplast was originally a cyanobacterium engulfed by another cell. (Similarly there is much evidence to suggest that the first mitochondrion was a bacterium engulfed by a cell. Topics to be covered elsewhere in this book!)
For whatever reason, perhaps from one or some of the ones above, cyanobacteria evolved green-reflecting chlorophyll pigments. If a cell did engulf a cyanobacterium, this only happened the once in all of evolution, as evidenced by the structural similarities of chloroplasts in all plants and algae. If so, then this cyanobacterium-chloroplast became the common ancestor of all plants and algae to come, all of which would take on the same green appearance ‘fixed’ in evolution as a ‘good enough’ solution.
About the Author
BSc(Hons), U.Syd. - double major in biochemistry and microbiology, with honours in microbiology
PhD, U.Syd - soil microbiology
Stumbled into IT and publishing of all things.
Discovered jujube trees and realised that perhaps I should have been an agronomist...
So I combined all the above passions and interests into this website and its blog and manuals, on which I write about botany, soil chemistry, soil microbiology and biochemistry - and yes, jujubes too!
Please help me buy a plant if you found this article interesting or useful!